DMC / WSU Neurointerventionalists Perform Ground-Breaking Embolization Procedure
On 11.12.2012, Dr. Sandra Narayanan, with the assistance of Drs. Samuel Tsappidi and Neelesh Nundkumar, performed the first Pipeline Embolization Device (PED) placement at the Detroit Medical Center. This procedure took place at Harper University Hospital and was a technical and clinical success.



The three physicians who were part of the first Pipeline procedure (from L to R):
Dr. Sandra Narayanan, Dr. Samuel Tsappidi, and Dr. Neelesh Nundkumar.
PED is a FDA-approved, flow-diversion treatment for large, wide-necked, fusiform, and/or recurrent intracranial aneurysms. The device is designed for parent vessel reconstruction, rather than endosaccular obliteration, and is a newer alternative to endovascular coil embolization or surgical clip ligation. A flexible, braided cylindrical mesh of 48 cobalt chromium and platinum tungsten strands, the PED provides 30-35% metal surface area, 3-5 times as much as conventional intracranial stents, resulting in more rapid and sustained intra-aneurysmal thrombosis and >95% aneurysm occlusion rates at 6 months.
"Therapeutic options for large and fusiform intracranial aneurysms are limited. While we continue to learn more about the diverse applications and long-term safety profile of the Pipeline device, it appears to be a very promising treatment for these challenging lesions," stated Dr. Narayanan.
Results of the PED system*
| A | B | C |
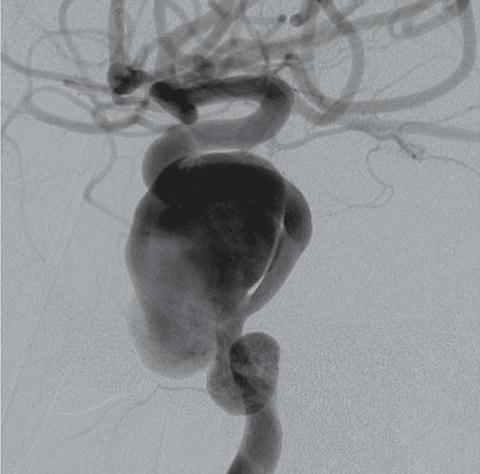


A: Pre-treatment angiogram: giant, dissecting cavernous ICA aneurysm
B: Immediately post-PED (subtracted image): contrast stagnation within aneurysm after PED placement
C: 6-month follow-up angiogram: good parent vessel reconstruction without residual opacification of aneurysm
*Images taken from Pipeline brochure, found at: http://www.ev3.net/assets/008/5898.pdf and reproduced with permission from Covidien.